Taysha Gene Therapies Announces First Pediatric Patient Dosed with TSHA-102
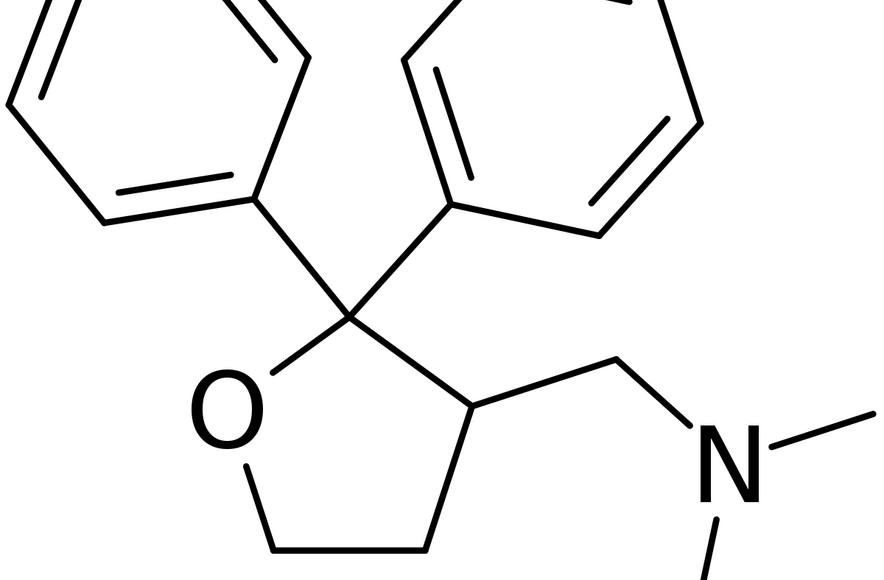
Taysha Gene Therapies, Inc. (Nasdaq: TSHA), a clinical-stage gene therapy company focused on developing and commercialising AAV-based gene therapies for the treatment of monogenic diseases of the central nervous system (CNS), today announced that the first paediatric patient has been dosed with TSHA-102 in the REVEAL Phase 1/2 paediatric trial…